Reduce PI Industries ltd For Target Rs.3,300 By Emkay Global Financial Services Ltd

Kumiai Chemical Industry (Kumiai) has given guidance for ~11% YoY decline in AXEEV (pyroxasulfone) sales for FY26. Kumiai’s AXEEV sales forecast at start-FY25 was a ~7% decline, although it ended the financial year with growth of ~6% on the back of higher sales in 1) US, led by reduction in distribution inventories and 2) Australia, led by legal action against generic manufacturers. We believe such inventory liquidation-led sales growth is largely behind now, and FY26 would be the first year of sales de-growth for Kumiai leading to lower offtake from PI before pricing falls and volumes grow. We model in de-growth of ~12% in PI’s sale of pyroxasulfone for FY26E (including contract assets) and ~10% sales CAGR over FY26E-28E, mainly led by volume growth as it nears patent expiry in US (seeing pressure from generics). We trim FY27E/28E EPS by 2-3%; retain REDUCE on PI, while revising down our TP by ~10% to Rs3,300. We lower the target multiple from 28x to 25x, factoring in the FY26 forecast by Kumiai and low visibility on scale-up of new molecules in CSM exports.
Kumiai’s FY26 earnings forecast indicates pressure on AXEEV from generics Kumiai’s revenue for AXEEV was JPY71.1bn in FY24, while it logged incremental sales worth JPY4.4bn in FY25 that led to JPY75.5bn of revenue; the mgmt now guides for a decline of JPY8bn that would result in lower sales—at ~JPY67bn (down ~11% YoY) in FY26. Kumiai’s aims to maintain price competitiveness as a countermeasure against generics, which would also result in a lower profitability forecast. Kumiai targets reducing production costs and negotiating with suppliers on lowering the cost of various raw materials, to improve its competitiveness. In our view, this will eventually flow down to suppliers like PI, making it necessary to optimize costs to protect their profitability.
AXEEV inventory level reducing with Kumiai; contract assets booked by PI Kumiai’s finished goods (FG) inventory has fallen from 170 days as of Oct-24 to 128 days as of Oct-25. This was primarily owing to liquidation of inventory in the US. Notably, the number of inventory days for PI increased to 59 as of Sep-25 vs 45 in FY25. We understand that PI is holding pyroxasulfone inventory and has recorded contract assets worth Rs8.5bn as of Sep-25. This indicates that FG produced by PI for Kumiai is under a binding agreement, with goods yet to be delivered – revenue has been booked, while deliveries are targeted for H2FY26. Hence, improved export shipment volumes in Q3 should not be interpreted as higher sales but will rather offset contract assets in our view.
PI’s Q2 commentary suggests macro challenges PI is cautiously optimistic for H2, backed by committed customer offtake. The macro environment remains uncertain, and PI expects volatility due to the geopolitical situation. The mgmt has given guidance for sales pickup from Q4FY26, with Q3 expected to be muted; however, structural recovery only from H2CY26 is in line with guidance. We build in a low single-digit dip in FY26 (-4% YoY for overall revenue; -8% for CSM exports).
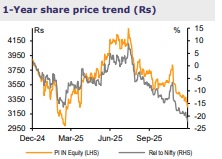
For More Emkay Global Financial Services Ltd Disclaimer http://www.emkayglobal.com/Uploads/disclaimer.pdf & SEBI Registration number is INH000000354