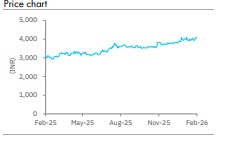
Accumulate Torrent Pharma Ltd For Target Rs.4,351 By Elara Capital

Strong and steady
Torrent Pharma (TRP IN) reported a steady Q3FY26, with revenue 2.2% above our estimates, while EBITDA, EBITDA margin and PAT were broadly in line with our expectations. Performance was driven by sustained double -digit growth in India and Brazil, alongside healthy traction in the US. Germany remained impacted by continued supply disruption at a third -party vendor . The acquisition of controlling stake of 48.8% in JB Chemicals & Pharmaceuticals (JB Pharma) has been completed on 21 Jan uary 20 26, with line -by -li ne consolidation effective Q4FY26. Management expects INR 4 .0– 4.5bn cost synergies in the next 2– 3 years (~20% in one year). While near -term integration may cause operational adjustment in Q4, fundamentals remain intact. We lower our FY26E & FY27 core EPS by 6-9% as we build in the impact of JB Pharma consolidation; we raise FY28E core EPS by 5%. We retain Accumulate with a higher TP to INR 4,351.
India outperforms IPM: India revenue grew 14% YoY, ahead of IPM growth, driven by strong chronic performance and volume -led growth . Cardiac, gastro and diabetes led therapy momentum, while Curatio sustained robust growth. Field force expansion continues, and we expect India to maintain market outperformance , supported by chronic portfolio scale -up and productivity gains.
JB Pharma acquisition to drive margin and EPS accretion: T RP has acquired a 48.8% controlling stake in JB Pharma , with consolidation effective from Q4FY26. Management has guided for cost synergies over the next 2 – 3 years, with ~20% likely in year one; these are entirely cost -led, with revenue synergies yet to be assessed. JB Pharma operates at a lower margin than TRP , offering scope for improvement post integration. With a 15 -year amortization policy and strong cash generation, we expect leverage to decline steadily in the next few years.
Brazil robust; Germany soft; US revival underway: Brazil delivered strong branded momentum with healthy double -digit growth and continued outperformance vs the market. Semaglutide filings are under priority review at ANVISA and remain a key medium -term trigger, although competiti on could lead to pricing pressure. The pipeline remains robust with multiple molecules under review. We expect TRP’s LatAm business to be a steady 10+% compounder . In Germany, performance continues to be impacted by third -party supply disruption, with alternate sourcing likely to take a few quarters. The US business showed gradual improvement, supported by recent launches gaining traction, with management targeting m eaningful scale -up over the next year , aided by a strengthened pipeline.
Retain Accumulate with a higher TP of INR 4,351: We lower our FY26E & FY27 core EPS by 6 - 9% as we build in the impact of JB Pharma consolidation; we raise our FY28E core EPS by 5% . TRP currently trades at 64.8x FY27E core P/E. Rich valuation is justified , we believe , by deleveraging -led faster EPS growth and significantly higher cash EPS. We retain Accumulate and raise our TP to INR 4, 351 from INR 4,137 on 52x FY28E core P/E plus cash per share. Prolonged Germany disruption, slower US ramp -up and JB Pharma integration risks are key risks.
Please refer disclaimer at Report
SEBI Registration number is INH000000933


















.jpg)


.jpg)