Buy Sun Pharma Ltd for the Target Rs.2,000 By Emkay Global Financial Services Ltd

While we had addressed concerns around the sustainability of Sun’s domestic outperformance in one of our earlier notes (link), we focus on the street’s concerns around Sun’s specialty investments in FY26 + new specialty launches undershooting medium-term street expectations. Even as we acknowledge that Abbvie’s superior Phase 3 trial read-out would establish Rinvoq as the standard of care for Alopecia Areata (AA) post-commercialization (launch + scale up, however, is at least 3 years away), our market share assumptions for Leqselvi were anyway conservative to begin with (~5% by FY30E, when we expect Leqselvi to post close to USD500mn in global sales; consensus estimate for Pfizer’s Litfulo in CY26—the 3 rd full year of sales—at ~USD240mn, despite subpar efficacy). We analyze the evolution of payor coverage for Plaque Psoriasis and Atopic Dermatitis in the US + the extant prior authorization policies for AA which lead us to believe that concerns around payor coverage for Leqselvi are overstated and will be addressed in the medium term. We note that the AA space in the US (no other candidate currently in Phase 3) is nowhere nearly as competitive as the Plaque Psoriasis space, where Ilumya has well exceeded street expectations despite multiple injectable as well as oral treatment options being available + a weaker efficacy profile + being a relatively late entrant. Besides, additional indications for Leqselvi (which will be a subset of Rinvoq’s approved indications) as well as Unloxcyt (same as Libtayo’s other approved indications) are currently not a part of our NPV estimates. We believe that comparisons of Sun’s current multiples with its 5-year average trading range are unjustified, given that the company’s revenue mix has meaningfully changed over FY20-25 (branded sales share at 70% in FY25 vs 56% in FY20; FY28E at 76%). Notably, while domestic formulations are one-third of the overall top line, the business accounts for ~55% of Sun’s overall EBITDA pool (which will continue to witness double-digit growth). We reiterate BUY.
Concerns around payor coverage for Leqselvi overstated
An analysis of the evolution of payor coverage for new treatment options for Plaque Psoriasis and Atopic Dermatitis in the US suggests that concerns around payor coverage for Leqselvi are overstated and will be addressed in the medium term. Insurers typically begin offering coverage with restrictive policies for 2-3 years post-launch, followed by relaxation of requirements and gradual expansion in coverage. Early biologics for Psoriasis such as Enbrel and Stelara faced hurdles, including strict prior authorizations and documentation of experience with unsuccessful conventional therapies. Similarly, Dupilumab (Dupixent)—approved for moderate-to-severe Atopic Dermatitis in CY17— faced restrictive initial coverage (limited to patients satisfying clinical criteria, which was more stringent than FDA’s inclusion criteria for clinical trials), which was subject to prior authorizations, appeals, and step therapy. Over time, coverage for both treatment options expanded owing to strong patient outcomes and increase in prescriber comfort.
While one could argue that coverage evolution for Alopecia Areata might not mirror that for Plaque Psoriasis/Atopic Dermatitis given that some plans currently exclude Alopecia, per the National Alopecia Areata Foundation in the US, ~40% of appeals against denied insurance claims are successful; also, patients who meet the clinical requirement can request for an external review by a state/federal agency, following which the probability of a denial is lower, given favorable precedents. In case of Leqselvi, the step therapy requirement (conventional systemic therapy/high-potency topical corticosteroid) has been waived off for patients already on Eli Lilly’s Olumiant and Pfizer’s Litfulo (we do expect Leqselvi to initially gain share from competing JAK inhibitors currently in the market, given its superior efficacy profile). The clinical requirement (at least 50% scalp hair loss for a minimum of 6 months) imposed by payors so far is also in line with the FDA’s inclusion criteria for clinical trials. Our view is also consistent with past studies, which indicate that coverage for specialty drugs in the US turns more favorable the longer these drugs are in the market.
Additional indications for Leqselvi and Unloxcyt currently not a part of our NPV estimates
Of the multiple other indications that JAK inhibitors have been approved for (prominent chronic inflammatory conditions, including Rheumatoid Arthritis, Psoriatic Arthritis, Juvenile Idiopathic Arthritis, Axial Spondyloarthritis, Ulcerative Colitis, and Atopic Dermatitis; rare forms of blood cancer, such as Myelofibrosis and Polycythemia Vera), Sun is likely to target 2-3 other indications with Leqselvi. Sun will target Atopic Dermatitis and Psoriatic Arthritis, in our view, given the synergies with its existing specialty brands and derma field-force. Notably, Sun has refrained from targeting indications such as Crohn’s disease and Ulcerative Colitis with Ilumya, unlike other IL-23 inhibitors, given the need to establish a dedicated gastro sales-force. Other JAK inhibitors, including Olumiant, Litfulo, and Rinvoq, are also being investigated for Vitiligo, an indication that Sun could also pursue with Leqselvi, given its settlement with Incyte (Incyte’s topical Ruxolitinib was approved for the treatment of nonsegmental Vitiligo by the FDA in CY22). Unloxcyt could also be evaluated for Libtayo’s other approved indications (Basal Cell Carcinoma and Non-Small Cell Lung Cancer). It is to be noted that Unloxcyt had demonstrated an overall response rate in line with that of Libtayo (albeit head-to-head comparison not appropriate) within the Non-Small Cell Lung Cancer cohort in Phase 1 trials.
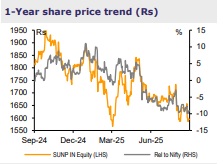
For More Emkay Global Financial Services Ltd Disclaimer http://www.emkayglobal.com/Uploads/disclaimer.pdf & SEBI Registration number is INH000000354








.jpg)