Astellas vs Lupin and Zydus in the Myrbetriq Legal Battle
* Astellas Pharma is the innovator of Myrbetriq, a treatment for overactive bladder, launched in 2012, which later became a blockbuster drug.
* In 2019, Lupin and Zydus Lifesciences filed ANDAs with the USFDA to market generic versions of Myrbetriq.
* Both companies have since faced patent litigation initiated by Astellas.
* In June 2023, the Delaware district court invalidated Astellas’ Mirabegron patent, because it was just using existing drug-making methods to solve a known problem (how food affects drug absorption), and that’s not considered a true invention under patent law.
* However, the US Court of Appeals vacated that decision in September 2024 and remanded the case for further proceedings.
* Despite the ongoing litigation, Lupin and Zydus launched their generics in the US after receiving FDA approval, as the court denied Astellas’ injunction request.
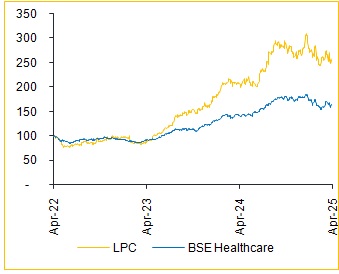
* A recent US court ruling favored Astellas, potentially affecting the commercial outlook for Lupin’s and Zydus’s versions.
As per IQVIA, Myrbetriq recorded U.S. sales of approximately USD 1.6 Bn as of July 2024
* Possible Scenarios for Zydus and Lupin to Navigate the Case
With ongoing litigation, Zydus and Lupin have two main strategic options, each with its own set of opportunities and risks:
* Pursue Ongoing Litigation and Appeal Decisions: Both companies can continue to challenge Astellas’ patent claims by appealing the recent ruling. If successful, they could secure a favorable decision allowing continued sales of their generic versions of Myrbetriq in the U.S. This option offers significant upside if they win but carries the risk of prolonged legal battles and costs if they lose. The appeal process could also delay a final resolution for months or even years.
* Explore Licensing or Partnership Opportunities: Given the litigation uncertainty, Zydus and Lupin could pursue licensing agreements with Astellas or other companies, allowing them to sell generics under mutually agreed terms, such as royalties or profit-sharing. This offers a more predictable revenue stream but may limit control over pricing and margins, and could reduce the revenue potential if the litigation is resolved in their favor
That said, our current forecasts do not factor in the potential upside from continued generic sales in the US, nor any associated legal penalties or fees due to the uncertainty surrounding the situation.
For Detailed Report With Disclaimer Visit. https://choicebroking.in/disclaimer
SEBI Registration no.: INZ 000160131