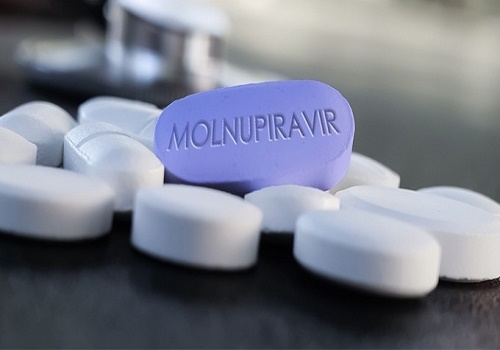
Covid antiviral drug Molnupiravir launched in India
Follow us Now on Telegram ! Get daily 10 - 12 important updates on Business, Finance and Investment. Join our Telegram Channel
Covid antiviral drug Molnupiravir was on Monday launched in India at Rs 1,399 for a five-day course for mild to moderate infection.
Amid rising cases of new Covid variant Omicron in the country, an expert panel of the Central Drugs Standard Control Organisation had recently approved antiviral drug Molnupiravir for restricted use in emergency situation.
Along with Molnupiravir, the CDSCO has also granted emergency use authorisation to the Serum Institute of India's Covid vaccine COVOVAX, Hyderabad based Biological E RBD Protein CORBEVAX.
Molnupiravir is an antiviral medication that inhibits the replication of certain RNA viruses. It is used to treat COVID-19 in those infected by SARS-CoV-2. The recommended dose of Molnupiravir 800 mg is twice a day for five days. A patient needs to take 40 capsules containing 200 mg of medication. Over a dizen pharma companies including Torrent, Cipla, Sun Pharma, Dr Reddy's, Natco, Mylan, and Hetero are in the process to manufacture the oral pill.
Cipla, Sun Pharma, and Dr Reddy's Laboratories are also expected to release Molnupiravir capsules in the coming weeks.
Molnupiravir, developed by MSD and Ridgeback Biotherapeutics, is also the first oral anti-Covid pill approved by UK's drug regulator. The US Food and Drug Administration (USFDA) has also cleared Molnupiravir for the treatment of mild-to-moderate Covid-19 in adults, and for those who are at a high risk to severe disease.
-